When you're running a clinical trial, not every side effect is a red flag. But figuring out which ones are? That’s where things get messy. Too many researchers mix up serious adverse events with severe ones-and that’s a costly mistake. The difference isn’t about how bad the symptom feels. It’s about what happens to the patient afterward. Get it wrong, and you’re drowning in paperwork that doesn’t matter. Miss it, and you’re putting lives at risk.
What Makes an Adverse Event "Serious"?
An adverse event (AE) is any unwanted medical occurrence during a clinical trial-whether it’s linked to the drug or not. But only some of those count as serious. The FDA and ICH E2A guidelines define seriousness by outcome, not intensity. Here’s the exact list:
- Death
- Life-threatening condition (the patient was at immediate risk of dying)
- Requires hospitalization or extends an existing hospital stay
- Results in permanent disability or significant loss of function
- Causes a congenital anomaly or birth defect
- Requires medical or surgical intervention to prevent one of the above
That’s it. No more, no less.
Let’s say a patient gets a bad headache during a trial. It’s pounding, they can’t focus, they take three painkillers. That’s severe-but not serious. Unless that headache leads to a brain bleed and emergency surgery? Then it becomes serious. The pain level doesn’t matter. The outcome does.
Another common confusion: psychiatric events. A participant reports "severe anxiety." If they’re just having panic attacks but can still go home and sleep? Not serious. But if they attempt suicide and need ER intervention? That’s serious. Same symptom. Different outcome. That’s the line.
What Counts as Non-Serious?
Everything else. If it doesn’t meet those six criteria, it’s non-serious. That includes:
- Mild nausea that goes away after a few hours
- A rash that itches but doesn’t blister or spread
- Temporary dizziness after standing up
- Mild fatigue that doesn’t stop someone from working
- Headaches that respond to over-the-counter meds
These still need to be documented-usually in Case Report Forms (CRFs). But they don’t trigger the rush of emergency reporting. They’re tracked over time, summarized monthly or quarterly, and reviewed by the Data Safety Monitoring Board (DSMB) as part of routine safety checks.
Here’s the key: severity ≠ seriousness. The Common Terminology Criteria for Adverse Events (CTCAE) grades symptoms from Grade 1 (mild) to Grade 5 (death). But a Grade 3 event-say, moderate liver enzyme elevation-isn’t automatically serious. If it doesn’t cause hospitalization or permanent damage, it’s still non-serious. Many sites get this wrong. In fact, a 2022 Advarra survey found that 63.4% of research sites had inconsistent seriousness judgments across different studies.
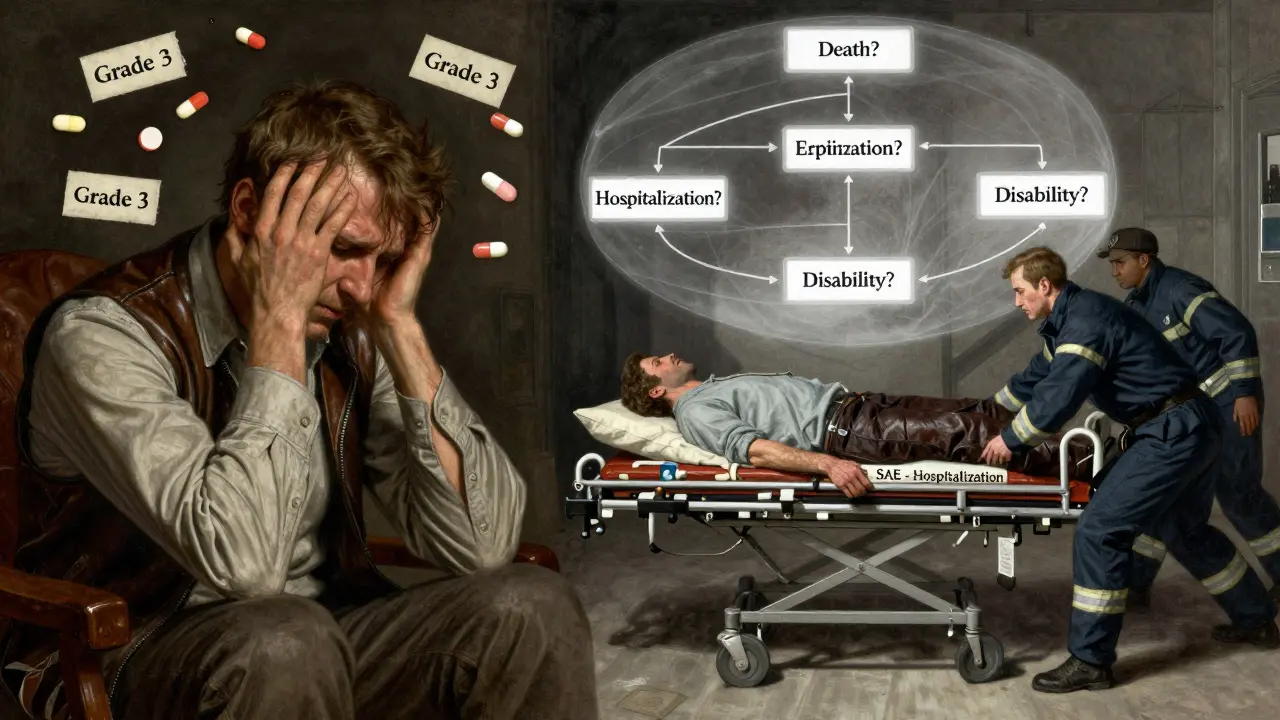
When Do You Have to Report?
Timing isn’t optional. It’s the law.
Serious adverse events (SAEs): Investigators must report them to the sponsor immediately. That means within 24 hours of finding out. Doesn’t matter if you think it’s related to the drug. Doesn’t matter if it’s the 3rd time it’s happened. Report it. The FDA’s 21 CFR 312.32 is crystal clear on this. The sponsor then has 7 days to report life-threatening SAEs to the FDA, and 15 days for others. If it’s unexpected and serious? Even faster.
IRBs (Institutional Review Boards) also need to know. Most require SAEs reported within 7 days. Some ask for them within 24 hours if the event is fatal or life-threatening.
Non-serious adverse events: These follow the protocol’s schedule. Maybe you report them every month. Maybe every quarter. Maybe not at all to the IRB-unless the protocol says so. They’re logged in the database, analyzed for patterns, and flagged if they start clustering. But they don’t stop the trial. They don’t trigger emergency calls.
Here’s what happens when you mess this up:
- You flood the system with noise. The FDA’s Sentinel system has processed over 14.7 million reports since 2008. Only 18.3% were serious. That means 82% were waste.
- You waste hours. SWOG Cancer Research Network found 31.8% of their SAE reports needed corrections because of misclassification. That’s 18.5 full-time hours per week lost.
- You miss real signals. When everyone’s screaming about mild rashes, the one patient with sudden kidney failure gets buried.
Why Do People Keep Getting It Wrong?
Because it’s not intuitive.
Doctors are trained to treat symptoms. If a patient has a severe cough, you treat it. So naturally, you think: "Severe = serious." But in clinical trials, severity is just one piece. Outcome is the whole puzzle.
And the pressure? It’s real. Clinical coordinators are overwhelmed. They’re juggling consent forms, lab results, scheduling. They don’t have time to re-read ICH guidelines. A Reddit thread from January 2023 had 147 comments from coordinators saying they were confused-especially with psychiatric events, pain, or fatigue.
Even big institutions struggle. At UCSF, 42.3% of AE reports in 2022 needed clarification. That’s nearly half. Each one delayed review by nearly 10 business days.
And it’s worse in oncology. Patients already have low blood counts, liver issues, nerve damage from chemo. Is that new numbness from the trial drug-or just progression? That’s why 78.2% of oncology sites had inconsistent seriousness ratings.

How to Get It Right Every Time
You don’t need to be a regulatory expert. You just need a system.
Use the NIA decision tree:
- Did the event cause death?
- Was it life-threatening?
- Did it require hospitalization or extend one?
- Did it cause permanent disability or significant incapacity?
If the answer to any is yes? It’s serious. Report it now.
Use the FDA’s MedWatch Form 3500A. It has checkboxes for each seriousness criterion. No guesswork.
Train your team. ICH E6(R2) says everyone involved must be trained before the trial starts. And it’s not a one-time thing. 98.7% of top 50 research sites require annual refresher training. If your site doesn’t? Fix it.
Use AI tools. Since 2020, automated seriousness classifiers have improved from 76% to 89.7% accuracy. They don’t replace judgment-they support it. They flag events that need a second look. They reduce errors before they hit the system.
And don’t forget: the EU’s Clinical Trials Regulation (2022) and the FDA’s 2023 draft guidance are pushing for more standardization. Soon, reporting will be even more uniform. But right now? You’re still on the front line. Get it right.
The Bigger Picture
Why does this matter beyond compliance?
Because patient safety isn’t a checkbox. It’s a rhythm. Every time you report a non-serious event as serious, you dilute the signal. Every time you miss a real SAE, you risk a patient’s life. The global market for clinical trial safety systems is worth over $3 billion-and 62.7% of that spending is wasted on misclassified events.
That’s not just inefficient. It’s dangerous.
When you report correctly, you help sponsors spot real safety signals faster. You help regulators make better decisions. You help future patients avoid harm.
It’s not about being perfect. It’s about being precise.
Is a severe headache always a serious adverse event?
No. A severe headache is only serious if it leads to death, hospitalization, permanent disability, or requires intervention to prevent one of those outcomes. If the patient takes ibuprofen and feels better the next day, it’s severe but not serious.
Do I need to report a mild rash to the IRB?
Usually not. Mild rashes that don’t require treatment, hospitalization, or cause lasting damage are non-serious. They’re documented in CRFs and reviewed during routine safety monitoring. Check your protocol-if it says to report all rashes, follow that. But standard practice is to report only serious events to the IRB.
What if I’m not sure whether an event is serious?
When in doubt, report it as serious. It’s better to over-report than under-report. But make sure you follow up with the sponsor or safety team to clarify. Many sites have a "gray zone" review process where safety officers assess borderline cases within 24 hours.
Can a non-serious adverse event become serious later?
Yes. An event that starts as mild-like a small fever-can progress to pneumonia and require hospitalization. If that happens, you must report it as a serious adverse event as soon as the outcome changes, even if it was originally classified as non-serious.
Are all deaths during a trial considered serious adverse events?
Yes. Any death occurring during a clinical trial, regardless of cause, is automatically classified as a serious adverse event. The cause must be investigated, but the event itself triggers immediate reporting.
How often should staff be trained on AE reporting?
At least annually. ICH E6(R2) requires documented training before a trial starts, and 98.7% of top research institutions require yearly refreshers. Training should include real case examples, the ICH E2A criteria, and updates to local protocols.


Write a comment